Hygienic and sanitary design is the integration of design and engineering with the full food and beverage production environment to minimize contamination risk and enable effective cleaning and sanitation.
1. Complete a Risk Assessment
The first step is to complete a comprehensive risk assessment to understand the risks that exist across the entire production process. Hygienic design principles do not only apply to the direct food contact surfaces; indirect contact surfaces, non-product contact surfaces and people all carry a risk of enabling cross-contamination.
The completed risk assessment should be readily available and regularly revisited. Risks can be categorized as:
Biological Hazards: Of utmost importance are the main food pathogens: Salmonella, Listeria, Campylobacter and Cronobacter sakazakii in dry infant formulations. Biological hazards also include pests and microbiology.
Chemical Hazards: Residues from cleaning chemicals or disinfectants.
Physical Hazards: Foreign bodies from the equipment such as lost nuts and bolts, plastic pieces, glass particles and pieces of worn-out elastomers.
2. Is my surface cleanable to a microbial/allergen-free level?
Processing equipment and food-contact surfaces must be constructed in such a way that enables effective and efficient cleaning for the full lifespan of the equipment. Surfaces should be designed in such a way to avoid any allergens or foodstuffs remaining after cleaning and prevent bacterial ingress, survival, growth and reproduction.
Surface finish is the allowable deviation from a perfectly flat surface that is manufactured. The parameter most commonly used for measuring general surface roughness is Ra. It is accepted that surface roughness for stainless steel surfaces should be less than 0.8 micrometres.
It is important to remember that whilst the surface finish is usually correct when equipment is new, over time the surface can become damaged by wear and tear, equipment repair and grinding and corrosion of the surface producing “pits”. Equipment should be regularly evaluated for compliance. Equipment that has become damaged can usually be recovered by re-surfacing and treatments such as grinding, polishing and passivation can be used to improve cleanability.
3. Are my materials suitable?
Materials used for the manufacture of food processing equipment must be completely compatible with the product(s) being made, the production environment, cleaning and disinfecting chemicals and the methods of cleaning.
Materials should have non-painted surfaces to prevent foreign body contamination and should not contain any substances capable of tainting food. Electrical components should have the appropriate IP rating.
In general, stainless steel types AISI-304 offer sufficient corrosion protection and widely used for neutral products. In a more acid or oxidative environment, AISI-316 or AISI-316L are applied.
Depending on the application, some plastics may have advantages over stainless steel such as lower cost and weight as well as better chemical resistance
4. Are my surfaces drainable?
Equipment should be self-draining to assure that liquid which can harbor and promote the growth of bacteria does not accumulate, pool or condense on the equipment.
The exterior and interior of all equipment should be self-draining, This means that no horizontal surfaces should be present and all surfaces require a minimum 3% slope.
5. Is my equipment accessible for cleaning, inspection and maintenance?
If the cleaning method is not CIP (cleaning-in-place), all parts of the equipment need to be readily accessible for inspection, maintenance, cleaning and disinfection without the use of tools.
Maintenance enclosures and human-machine interfaces (HMI) such as push buttons, valve handles, switches and touchscreens must be designed to ensure that food product, product liquid or water does not penetrate or accumulate in and on the enclosure or interface. The design of all enclosures should be sloped or pitched to negate the possibility of being used as a storage/shelving area.
Good hygienic and sanitation design also applies to pipework and nozzles used in automated and manual cleaning processes and does not stop there. The outside of machines needs to be cleaned effectively too.
6. Are the materials correctly sealed or properly welded?
Continuous welding is the preferred technique for the fabrication of stainless steel components. From a quality perspective, poor welds have much the same effect as poor surface finish. Poor welding typically leaves an excess of weld material on the surface (overfilled) or too little (undercut). Evidence of poor welding on the outside of a pipe is almost a sure sign of poor welding inside. All welds should be inspected internally minimally by an endoscope and in some cases, x-ray inspection is required.
7. Are there crevices or dead legs?
A process pipework dead leg is any “T” piece where the tee pipe length is more than half a pipe diameter in the “non-flow” direction or 1 pipe diameter in the “flow” direction. If the dead leg exceeds these dimensions there will not be sufficient CIP mechanical action in the dead leg to remove soiling and the disinfectant may not be able to contact the surfaces during the disinfection step, causing contamination of the next product processed.
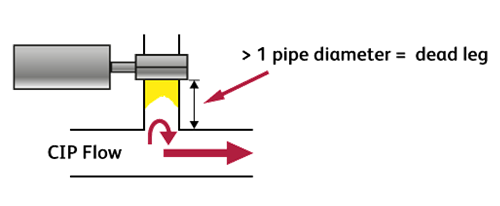
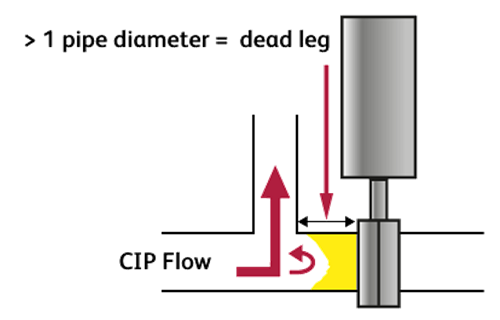
If the dead legs are excessively long or configured downwards, then they may remain full of “stagnant” product.
8. Are there hollow bodies?
Hollow areas of equipment such as frames and rollers must be eliminated wherever possible or permanently sealed. Bolts, studs, mounting plates, brackets, junction boxes, nameplates, end caps, sleeves and other such items must be continuously welded to the surface not attached via drilled and tapped holes.
9. Are cross-contamination vectors excluded?
Machinery must be so designed and constructed that no ancillary substances (e.g. lubricants, etc.) can come into contact with foodstuffs. They have to be resistant to cracking, chipping, flaking and abrasion. Open containers should be shielded.
Building construction and zoning principles should be understood and implemented and include several elements:
- The design and layout of workspaces allowing for one-direction production flow
- Sufficient lighting especially in food preparation and inspection areas to aid visual inspections during manual cleaning
- Suitable employee facilities such as non-touch hand-washing systems, door-fogging and break areas
- The materials and design of external and interior construction of walls, floors, doors.
- Physical separation of activities to prevent cross-contamination with allergens or biological hazards such as harmful microorganisms from raw to cooked food.
10. Is my cleaning documented, inspected and validated?
Procedures for cleaning and disinfection must be clearly written, designed and validated to be effective and efficient. Chemicals recommended for cleaning and disinfection must be compatible with the equipment and the manufacturing environment.

